Abstract
Background: Self-reported financial hardship (FH) amongst cancer patients is increasingly becoming a challenge for patients, caregivers, and healthcare providers. FH not only leads to financial struggles, significant lifestyle changes, and emotional distress, but also contributes to treatment noncompliance, affecting clinical outcomes. As treatment costs rise, it is crucial to develop efficient methods to proactively identify and alleviate FH in hematology practice. One potential approach is utilizing automated processes to identify those at highest risk of FH. At Mayo Clinic, screening for FH involves using a single financial strain question 'How hard is it for you to pay for the very basics like food, housing, medical care, and heating?' which all cancer patients answer annually as part of the institution's Social Determinants of Health (SDOH) assessment. Answers are on a five-point scale including not hard at all, not very hard, somewhat hard, hard, and very hard. In this study, we assess the prevalence and predictors for FH (denoted by a response of "Very hard" "Hard" or "Somewhat hard") amongst the Mayo Clinic hematologic malignancy patient population. Our study objective was to determine if this automated process could identify those at risk for FH.
Methods: Patients who received care for hematologic malignancies (lymphoma, leukemia, plasma disorders, myelodysplastic/myeloproliferative disorders, and other heme malignancies) at any of the Mayo Clinic cancer centers (Minnesota, Arizona, and Florida) and who had completed the SDOH screen at least once were included in this study. The electronic medical record (EMR) and Mayo Clinic Cancer Registry were utilized to extract demographic and disease variables. Patient's home zip code was used to determine rural/urban residence, distance from cancer center, and the Area Deprivation Index (ADI), a measure of socioeconomic disadvantage based on home zip code (ranging from 1-100, with 100 representing the most disadvantaged). Multivariable logistic regression modeling was used to examine predictor variables for FH in this patient population.
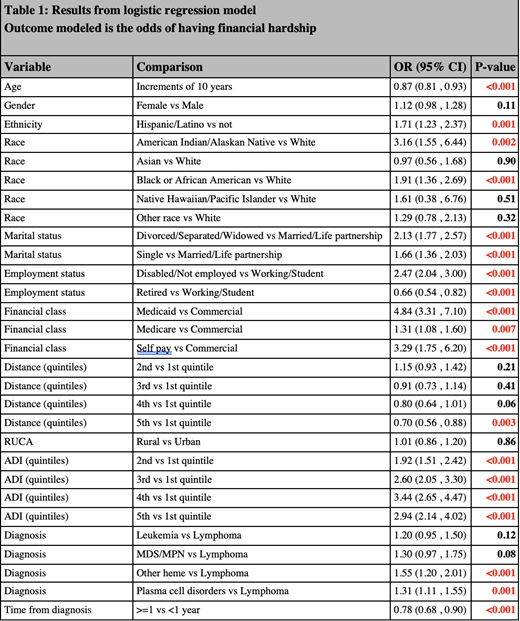
Results: The final cohort included 10,024 patients from 2018 to 2020. Median age was 64.6 years (IQR 58.1,73.7), 58% were male, and 79% married. Race/ethnicity composition was 94% White (n=9,268), 2.5% Black (n=246), 0.4% American Indian/Alaskan Native (44), and 4% Hispanic (n=360). Fifty-six percent of patients had Medicare and 41% had commercial insurance. Fifty percent were retired, 40% were working/students, and 72% were urban residents. Mean ADI was 41.2. Fifty-six percent of patients had lymphomas, 23.5% had plasma cell disorders, 8.5% had leukemias, 6.8% had other hematological malignancies, and 5.5% had myelodysplastic/myeloproliferative neoplasms. FH was reported by 12.8% (n=1286) of the patients. Table 1 shows the results of the multivariable model. A significantly higher likelihood of endorsing FH was noted in Hispanic vs non-Hispanics, Black and American Indian/Alaskan Native groups vs whites, Disabled/Unemployed vs working, Medicaid, Medicare, and Self-Pay groups vs commercial insurance, higher ADI (5 th quintile vs 1 st), and myelodysplastic/myeloproliferative disorder and other hematologic malignancy vs lymphoma patients. Older age, being retired, and living farther from the cancer center were associated with significantly less likelihood of endorsing FH.
Conclusion: Our study used automated data extraction from the EMR to efficiently identify predictors of FH in hematologic cancer patients. Employing a dichotomized and automated "flag" for FH, particularly if incorporated in the EMR, could ease the identification of SDOH issues, facilitate timely connection to appropriate resources, and help provide better patient-centered care.
Ailawadhi: Sanofi: Consultancy; Cellectar: Research Funding; Karyopharm: Consultancy; Ascentage: Research Funding; Genentech: Consultancy; Janssen: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Beigene: Consultancy; GSK: Consultancy, Research Funding; AbbVie: Consultancy; Medimmune: Research Funding; Pharmacyclics: Consultancy, Research Funding; Takeda: Consultancy; Amgen: Consultancy, Research Funding; Xencor: Research Funding. Fonseca: OncoTracker: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy; BMS: Consultancy; Mayo Clinic in Arizona: Current Employment; Aduro: Consultancy; AbbVie: Consultancy; GSK: Consultancy; Merck: Consultancy; Juno: Consultancy; Scientific Advisory Board: Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Patent: Prognosticaton of myeloma via FISH: Patents & Royalties; Novartis: Consultancy; Bayer: Consultancy; Celgene: Consultancy; Caris Life Sciences: Membership on an entity's Board of Directors or advisory committees; Kite: Consultancy; Janssen: Consultancy; Amgen: Consultancy; Pharmacyclics: Consultancy; Sanofi: Consultancy. Griffin: Exact Sciences: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal